Accelerate your product’s journey to patients with the support of our global network of scientific and technical experts. We know that time is life and expertise is key, so join our experts at CPHI Milan 2024 to find out how to overcome your drug development and manufacturing hurdles with an altogether different CDMO.

We work closely with you throughout the synthesis of your API and biologic drug substance, implementing operational excellence across our entire process.
We have a roster of analytical chemists, organic chemists and engineers who are adept at developing and trouble-shooting analytical methods and processes and have the expertise to help scale up your API process.
Therapeutic protein and antibody production including vaccine drug substance, monoclonal antibodies (mAbs), antibody drug conjugates (ADCs) and mAb biosimilars.
Helping you with a streamlined supply of plasmids and drug substances through to the creation of drug products.
From pellets, tablets and capsules to innovative release profiles, we have the global expertise to help meet your needs across a wide range of platforms and technologies.
From liquid and lyophilization to pre-filled syringes, vials and cartridges - helping you unlock the potential of your sterile injectable.

We leverage cutting-edge development and commercial manufacturing solutions to offer contract development & manufacturing services from small to large scale for oral solids, sterile injectables, small molecules and biologics; as well as regulatory services.
We are ready to be your strategic partner to help you change patients' lives - because Time is Life.
We bring high quality products from development to commercialization, with a reliable supply chain to help get your medicines to patients fast.

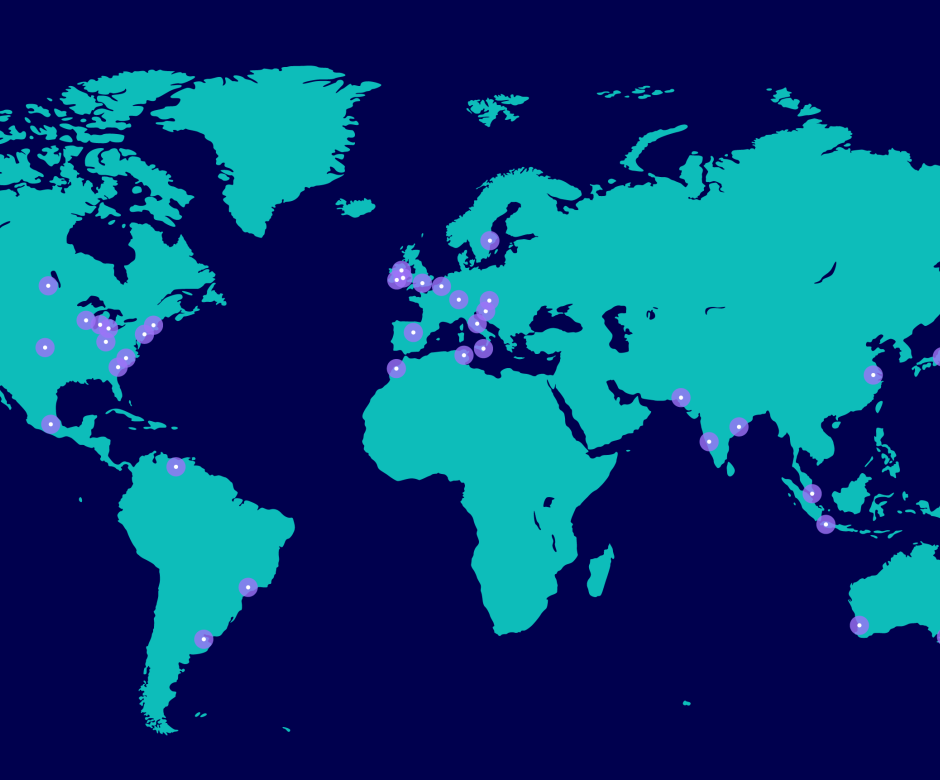
Easy for you to partner with our Pfizer global network of scientist and technical experts ready to meet your needs, working across our 35+ global sites.

Digital and IP protection for your product and business. Protecting your IP is at the core of what we do, with robust security programs in place.
Guided by our core values of courage, excellence, equity, and joy, we were honoured to receive the Outsourced Pharma & Life Science Leaders CDMO Leadership Awards in all categories for the eighth consecutive year, based on customer feedback.
This year, we were even named category champion in all areas and recognized for exceeding customer expectations in capabilities, compatibility, quality, reliability and service.
We thank our customers for recognizing our team’s dedication and skills, and look forward to continuing our joint efforts to accelerate drug development and bring medicine to patients faster because time is life.

Every drug we manufacture benefits from the expertise and world-class facilities of a parent company with over 170 years experience and hundreds of products to its name. Pfizer invests more than $1B into manufacturing to continuously improve infrastructure and processes.
Our global manufacturing network, supporting CDMO services, includes more than 35 sites across six continents, serving customers around the world. From these facilities, we deliver with genuine empathy and assurance. An altogether different kind of CDMO.
"Pfizer CentreOne has helped us at every step in the synthesis, from providing the initial intermediate through refinement of the steps leading to the API. We have faced a number of challenges with the manufacture of the API and the team has come up with creative solutions that show real scientific flair."
Michael Zasloff, MD PHD, Co-Chairman and CSO of Enterin
"If you have a medicine you’d like to get to market – and you want to see it generate revenue --- you might be better off with Pfizer."
12th Edition of the Pharmaceutical Innovation and Invention Index
"The wealth of experience in handling the two active constituents of our drug, Deflexifol™, is really important, as are the advantageous capabilities that Pfizer CentreOne brings to this collaboration...We are delighted to tell our stakeholders that we are working with Pfizer CentreOne. Their world-class expertise and capabilities in the commercial manufacture of cytotoxic drugs, and reputation for excellence de-risks Deflexifol™ development."
Dr. Christian Toouli, CEO & MD of FivepHusion
"I've been doing business with Pfizer for a number of years. Over the years Pfizer CentreOne has gone above and beyond to make sure Sparhawk has received raw material for my production needs. Notably this year during the pandemic I noticed little to no disruption in service. If all of our suppliers were this attentive, my job would be much easier. Keep up the good work and as always, thank you."
Bert Hughes, President, CEO Sparhawk Labs